
© Matej Meza / Universität Bremen
Atoms and Defects: A Visit to the Crystallography Lab
Geoscientist Ella Schmidt and chemist Thorsten Gesing open their laboratory doors. Using new methods, they are investigating the atomic structures of crystals.
Some people think of shiny colorful stones found in jewelry when they hear the word “crystals.” Others have magical stones with healing powers in mind. However, crystals are more – they can soften and decontaminate water, they make bricks fireproof, and they are used as catalysts in oil refineries. Geoscientist Professor Ella Schmidt and chemist Professor Thorsten Gesing are well aware of their properties and potential. In their respective research groups in faculty 5 and faculty 2 at the University of Bremen, they are active in the field of crystallography and have a special project. Using new methodologies, they are looking at what influence disorder has on the properties of crystalline materials – atom by atom. Their research can be used to understand how battery materials behave during charging and discharging or how impurities can be removed from water.
Atom by Atom
Those who still remember their chemistry lessons may still be familiar with the so-called structural models and know that they follow a scheme: Next to the blue atom is the red one, followed by another blue one. “However, many materials have defects in their arrangement that have little effect in small amounts. However, if a defect repeats itself, for example a defect or void already exists in less than one percent of the structure, this has an impact on the material,” explains Thorsten Gesing with one such school model in his hand. From a research point of view, things get interesting when the distribution of the atoms is actively influenced and these voids are distributed in a targeted manner. By changing the chemical composition under the influence of different temperatures, the solid-state chemist and his research group are investigating how this changes the atomic structure of the crystals. “Let’s take Lego bricks as an example: We have a material structure of red and blue Lego bricks and add yellow, green, and purple ones into the empty spaces – our defects. What happens to the material then? Can I even produce a completely different material with different properties as a result?” That is how the professor of solid-state chemistry, who has been working at the University of Bremen since 2011, describes his work.

© Matej Meza / Universität Bremen
Finding Out Where There Is Nothing
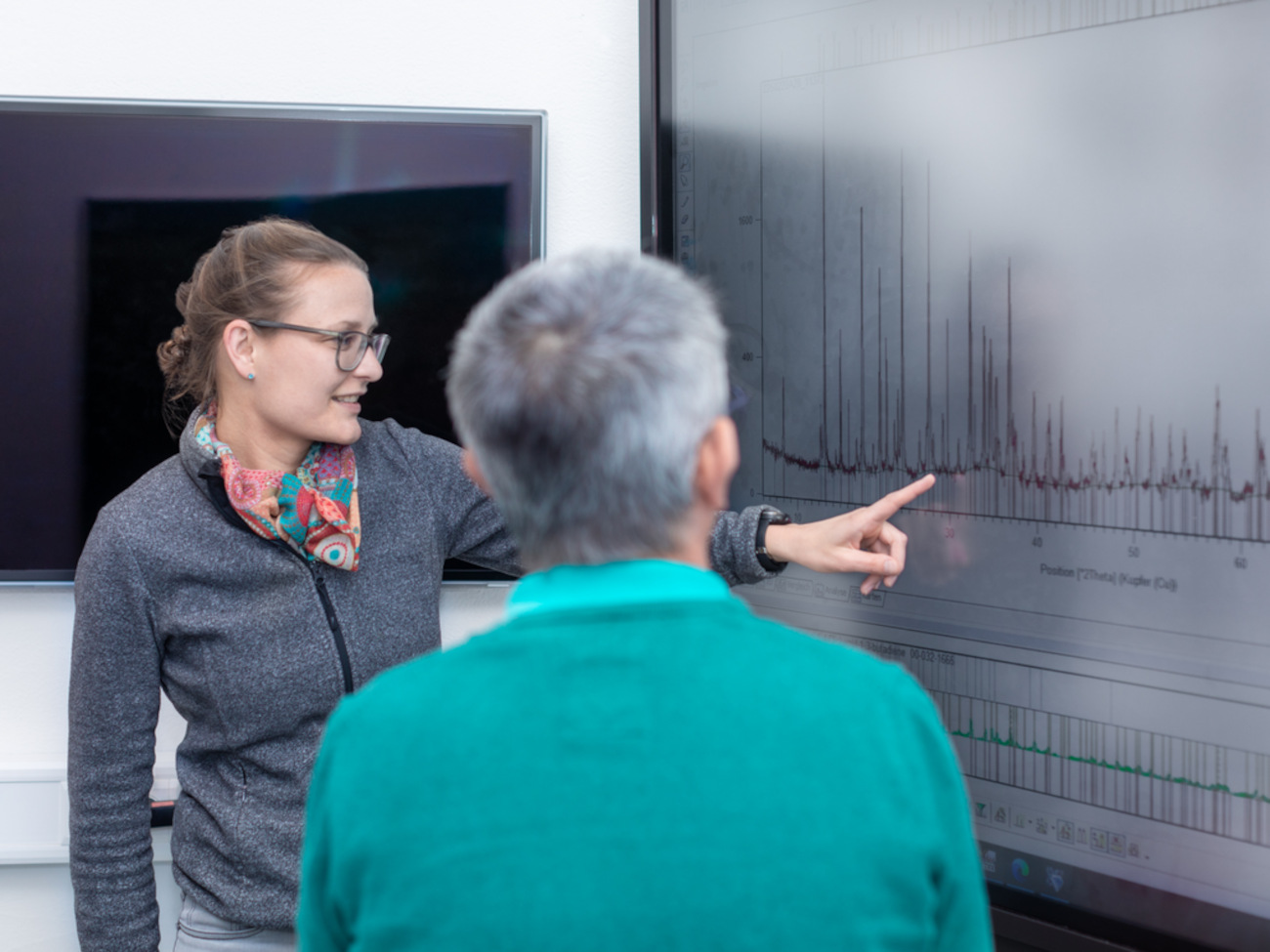
These modifications of the atomic crystalline structures are investigated with the help of different types of radiation. X-rays in a so-called diffractometer, for example, help to find the position of individual atoms and to distinguish between them. Infrared heat radiation shows the movement of the atoms together with their direct neighbors. Laser beams in a so-called Raman spectrometer can be used to see how well the arrangement of the atoms has formed and what deviations there are. The big challenge, however, is how to determine where there is nothing. That’s where Ella Schmidt comes in. An assistant professor of geosciences at the University of Bremen since February 2022, she is applying a new method to identify these voids. “Normally, X-ray diffraction is used as a method to determine the global atomic arrangement, where X-rays can be used to determine the atomic structure of a substance or even several substances. This method is used in the pharmaceutical industry, for instance when the purity of a substance needs to be determined. Spectroscopy is then normally used to determine the local order, i.e. a section within the atomic structure,” says Ella Schmidt, explaining the usual procedure.

© Matej Meza / Universität Bremen
However, the assistant professor is doing what is actually impossible: she is developing methods that can be used to determine the local order using X-ray diffraction. In this way, defects in the atomic structure can be identified more precisely than with spectroscopy. To the uninitiated, the result looks like a simple bump in a graph. To Ella Schmidt, it is much more. Based on these peaks in specific sections on the so-called diffraction diagram, she can determine the local order and thus identify the voids.
© Matej Meza / Universität Bremen
Interdisciplinary Research
Once Ella Schmidt has identified the void, Gesing can proceed with the chemical part, synthesizing and modifying the crystal to see if the added atoms fit into the void and have other effects on the atomic structure. Thus, the two research groups from both geosciences and chemistry are working hand in hand on foundation research to move closer to their goal. Geoscientist Ella Schmidt explains: “We normally start with the question of what material with what properties we should take so that we get a certain effect. One example is the production of fuel cells, which in the long term will replace the heavy batteries in cars and make it possible to drive 1000 km without refueling for a long time. What’s more interesting for us is whether and how we can modify the existing material so that it ends up with the desired properties.”

© Matej Meza / Universität Bremen
Incidentally, anyone expecting to find large, colorful crystals like those found in esoteric stores in Ella Schmidt’s and Thorsten Gesing’s laboratories will not find them in the crystallography department at the University of Bremen. The largest one is half an index finger long and that’s all that is needed for researchaa, as Thorsten Gesing explains: “Most materials are studied as powders or tiny crystals, since X-ray diffraction only requires crystals as large as a wafer-thin glass tip.”